Get more news. Sign up to our newsletter
Subscribe to our monthly News Digest and receive the latest news on the field of natural health products across different product categories:
- Food supplements
- Natural OTC / THMPs
- Medical devices
- FSMPs
and covering topics from regulatory to business insights.
The latest news from a&r
Dissent about the regulatory classification of food cultures
As is often the case in food manufacturing, food constituents can exert more...
How the EU will tackle greenwashing – Proposal on the Green Claim Directive
greenwashing
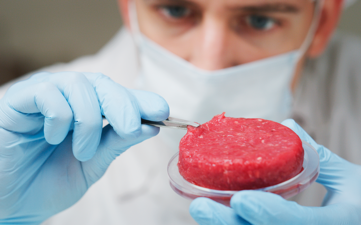
Italy plans the prohibition of cultured meat
The bill, which contains provisions banning the production and marketing...
Once again strict limitations for the interpretation of FSMPs by the CJEU
On March 2, the Court of Justice of the European Union (CJEU) issued...
MDR transition period and deletion of the MDR/IVDR ‘sell-off dates’ officially implemented
Regulation (EU) 2023/607 of the European Parliament and of the Council of 15...
Progress in the authorisation of novel foods and a cholesterol lowering health claim
Latest novel food authorisations In March 2023, the European Commission (EC)...
Botanical extracts are increasingly under pressure due to growing safety concerns
Recently, various botanical preparations derived from traditional ayurvedic...
Novel food status of biotechnically produced sodium hyaluronate clarified in consultation request
The consultation request under Article 4 of Regulation (EU) 2015/2283 concerns...
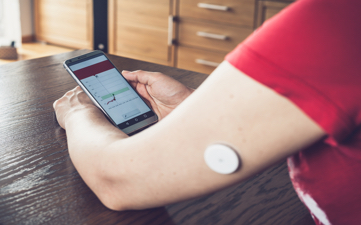
Advantage for Sponsors to utilize digital subject reporting outcomes (eDiary) in clinical trials
The past couple of years brought new challenges to clinical trials; however,...
Get in touch with us
![]()
We’d love to hear from you. Here’s how you can reach us: