Sustainability is all the rage these days, and more and more products are...
Regulatory
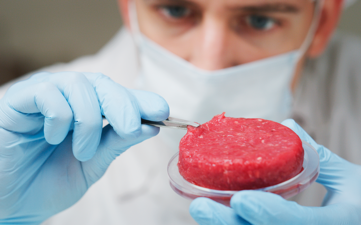
Italy plans the prohibition of cultured meat
The bill, which contains provisions banning the production and marketing...
Once again strict limitations for the interpretation of FSMPs by the CJEU
On March 2, the Court of Justice of the European Union (CJEU) issued...
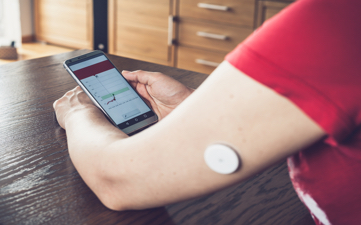
MDR transition period and deletion of the MDR/IVDR ‘sell-off dates’ officially implemented
Regulation (EU) 2023/607 of the European Parliament and of the Council of 15...
Progress in the authorisation of novel foods and a cholesterol lowering health claim
Latest novel food authorisations In March 2023, the European Commission (EC)...
Botanical extracts are increasingly under pressure due to growing safety concerns
Recently, various botanical preparations derived from traditional ayurvedic...
Novel food status of biotechnically produced sodium hyaluronate clarified in consultation request
The consultation request under Article 4 of Regulation (EU) 2015/2283 concerns...
EFSA’s work on the update or rather reduction of ULs
EFSA is in the process of revising the safety limits for certain vitamins and...
Medical Device Regulation Extension of transition period approved, but what are the implications?
In recent months, news about the Medical Device Regulation has accumulated....